
Zachary Schultz
Professor and Vice Chair of Graduate Studies
3033C McPherson Laboratory
140 W 18th Ave
Columbus, OH 43210
Areas of Expertise
- Analytical
Biography
Zachary D. Schultz earned his B.S. in Chemistry from the Ohio State University in 2000 and Ph.D. in Chemistry from the University of Illinois at Urbana-Champaign in 2005. He performed his doctoral studies under the supervision of Prof. Andrew Gewirth using infrared-visible sum frequency generation spectroscopy to characterize electrochemical interfaces. Prof. Schultz was a National Research Council Postdoctoral Fellow at the National Institute of Standards and Technology (USA). His research at NIST was performed largely in collaboration with Ira Levin at the National Institutes of Health (USA). Following his postdoctoral training at NIST, Dr. Schultz continued as a research fellow with Dr. Levin at NIH using vibrational spectroscopy and microscopy to study biomembrane systems. Prof. Schultz began his independent career as an assistant professor of chemistry and biochemistry at the University of Notre Dame in 2009, and was promoted with tenure to associate professor in 2015. In January of 2018, Prof. Schultz moved his research program to Ohio State. He was promoted to professor in 2022. Prof. Schultz has been recognized as a Cottrell Scholar, a Fellow of the American Association for the Advancement of Science and awarded the Craver Award by the Coblentz Society.
Research Overview
In the Schultz Lab, we believe the new scientific breakthroughs will be enabled by state of the art chemical measurement. Our research focuses on developing new tools for identifying molecules relevant to biomedical diagnostics and other applications. To do this, we build and develop instrumentation that takes advantage of chemical properties to characterize complex samples. The interaction between lasers and molecules provides unique information for detecting and identifying the components in complex systems. Understanding the basic science involved in chemical detection and manipulating these interactions has led to breakthrough technologies with tremendous potential. Much of the work in our lab involves vibrational spectroscopy, and in particular, Raman spectroscopy. We commonly leverage nanoparticles to enhance the signal, an effect commonly referred to as surface enhanced Raman scattering (SERS).
RAMAN IMAGING
The chemical specific Raman signal arising from the unique structure of molecules enables us to image chemical changes on the micrometer length scale. We have used Raman imaging to explore chemical uptake in live cells as well as monitor chemical changes in samples. In particular, our lab is interested in developing Raman imaging technology to increase spatial resolution, chemical information, monitor dynamic processes.
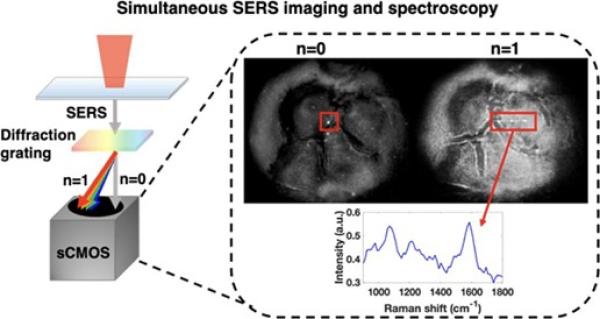
- Super Resolution SERS Spectral Imaging. Fluctuations in the SERS signal from nanoparticles enable localization microscopy to be applied to SERS imaging, providing spatial resolution of a few nanometers. We have developed spectral SERS imaging that provides both the position and chemical spectrum. We are applying this technology to understand fundamental interactions between molecules and nanostructures as well as improve spatial resolution and sensitivity in live cell imaging experiments.
- Multimodal Imaging. Coupling the chemical signals from Raman scattering with other imaging methods can increase understanding of the spatial activity of molecules. Techniques that we investigate in tandem with Raman include photothermal imaging, atomic force microscopy, second harmonic generation, and other nonlinear imaging modalities.
CHEMICAL DETECTION IN FLOW
We are exploring the use of surface enhanced Raman detection with online chemical separations such as: capillary electrophoresis, liquid chromatography, and other sampling techniques. Advances in instrumentation will enable new chemically selective methods for monitoring biological and other samples. Our lab invented and is developing sheath-flow surface enhanced Raman scattering, which uses the unique pattern of scattered light associated with the structure of molecules, to improve molecular identification. Applications include metabolomics and virus detection.
- Metabolomics: The ability to specifically identify and quantify the 40,000+ metabolites in the human body is essential for a systems biology approach to health care. Changes in biochemical pathways can be distinguished provided enough intermediate molecules can be monitored accurately. Current technologies can identify about 20% of the in biological samples. Sheath-flow SERS is orthogonal to existing technologies and should extend coverage of the number of detectable metabolites. In addition to identifying specific metabolites, the chemical rich signal can provide an indicator of metabolic phenotype.
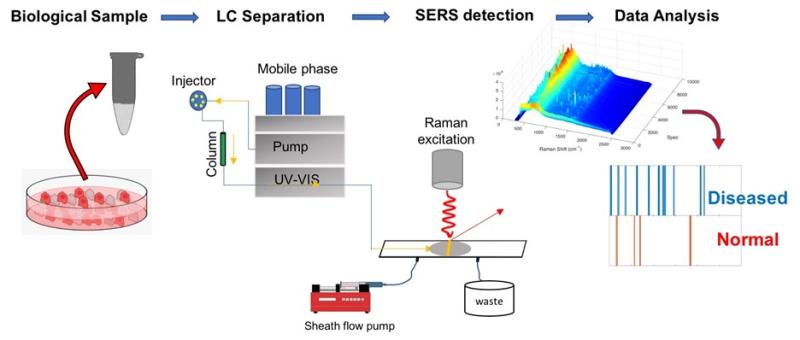
- Virus Identification. The unique molecules on the surface of viruses and other pathogens can be used to identify and quantify them. We are exploring the chemical signatures on these samples to improve quantifying virus titer and identifying signatures of infectious species.
ACTIVE PLASMONICS
Underlying all the problems we investigate is the basic science relevant to the signal enhancements incorporated into our measurements. We are interested in understanding how nanomaterials with plasmonic properties interact with light, particularly with respect to how these properties affect nearby molecules. This basic science serves as the basis for the development of future measurement techniques and other applications, such as photo-catalysts for CO2 reduction and selective protein detection in diagnostics.
Selected Publications
Scarpitti, B. T.; Chitchumroonchokchai, C.; Clinton, S. K.; Schultz, Z. D., In Vitro Imaging of Lycopene Delivery to Prostate Cancer Cells. Anal. Chem. 2022, 94 (12), 5106-5112.
de Albuquerque, C. D. L.; Zoltowski, C. M.; Scarpitti, B. T.; Shoup, D. N.; Schultz, Z. D., Spectrally Resolved Surface-Enhanced Raman Scattering Imaging Reveals Plasmon-Mediated Chemical Transformations. ACS Nanosci Au 2021, 1 (1), 38-46.
Payne, T. D.; Klawa, S. J.; Jian, T.; Kim, S. H.; Papanikolas, M. J.; Freeman, R.; Schultz, Z. D., Catching COVID: Engineering Peptide-Modified Surface-Enhanced Raman Spectroscopy Sensors for SARS-CoV-2. ACS Sensors 2021, 6 (9), 3436-3444.
Xiao L, Wang C, Dai C, Littlepage LE, Li J, Schultz ZD. Untargeted Tumor Metabolomics with Liquid Chromatography-Surface-Enhanced Raman Spectroscopy. Angewandte Chemie. 2020;59(9):3439-43. doi: 10.1002/anie.201912387
Landaeta E, Masitas RA, Clarke TB, Rafacz S, Nelson DA, Isaacs M, Schultz ZD. Copper-Oxide-Coated Silver Nanodendrites for Photoelectrocatalytic CO2 Reduction to Acetate at Low Overpotential. ACS Applied Nano Materials. 2020;3(4):3478-86. doi: 10.1021/acsanm.0c00210.
Sloan-Dennison S, Schultz ZD. Label-free plasmonic nanostar probes to illuminate in vitro membrane receptor recognition. Chemical Science. 2019;10(6):1807-15. doi: 10.1039/c8sc05035j
