
Casey Wade
Associate Professor
4105 Newman & Wolfrom Laboratory
100 W 18th Ave
Columbus, OH 43210
Areas of Expertise
- Inorganic
Bio
Casey Wade received his B.S. in Chemistry from the University of Nebraska-Lincoln in 2006. He completed his Ph.D. at Texas A&M University in 2011 where he studied the chemistry of boron and antimony Lewis acids under the supervision of Prof. François Gabbaï. Casey then moved to the Massachusetts Institute of Technology (MIT) to carry out postdoctoral research on the synthesis and applications of metal-organic frameworks with Prof. Mircea Dincă. Casey started his independent career as an assistant professor at Brandeis University in 2013 and joined The Ohio State University Department of Chemistry and Biochemistry in January 2018.
Research Overview
Research in the Wade Lab resides at the interface of molecular inorganic/organometallic chemistry and materials science and focuses on the design of new materials for catalysis and molecular separation. Current projects involve the synthesis and study of metal-organic framework (MOFs) containing organometallic catalyst sites and the design of bio-inspired MOF adsorbents for trace CO2 capture. Synthesis plays a central role in our research program, and a variety of solution and solid-state characterization techniques are used to elucidate the structure and properties of new molecules and materials. These include X-ray diffraction, gas porosimetry, thermogravimetric analysis, ICP-OES, cyclic voltammetry, and NMR, IR, and UV-Vis spectroscopies.
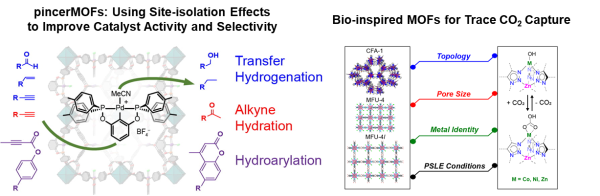
Site-isolation Effects in Metal-Organic Framework Catalysts
Advances in organometallic catalysis have had a profound impact on chemical synthesis in both academic and industrial settings. However, homogeneous organometallic catalysts suffer drawbacks related to stability, product separation, and recyclability. MOFs have emerged as versatile platforms for the design of heterogeneous catalysts that retain many of the beneficial features (e.g. ligand tunability) of homogeneous systems. In addition, catalyst immobilization can improve lifetime and activity via site-isolation effects. In this project, we are incorporating well-defined organometallic species into MOFs to study the effects of reactive site confinement on small molecule activation processes and catalytic processes such as Lewis acid activation, hydrogenation, and C–H functionalization. We have pioneered the design of “pincerMOFs” assembled from linkers based on transition metal diphosphine pincer complexes. These materials show significant differences in catalytic activity and selectivity compared to homogeneous analogues and have a broad scope of potential applications in catalysis.
Bioinspired MOFs for Trace CO2 Capture
The effect of CO2 on climate change and its potential for use as a renewable chemical feedstock have become strong motivating factors to develop new technologies for CO2 capture and conversion. Technologies that enable efficient capture of CO2 from the atmosphere are highly desirable since they allow remediation and utilization efforts to be distributed away from point sources such as power plants. Current strategies for CO2 removal from air such as aqueous amine scrubbing and causticization with metal hydroxide salts are very energy intensive and may not be economically viable. Solid adsorbents capable of reversibly extracting CO2 from air have the potential to be much more energy efficient and profoundly affect how we address the remediation and utilization of atmospheric CO2. In this project, we are using design strategies inspired by metalloenzymes to develop new MOF adsorbents for CO2 capture under atmospheric conditions. For example, MOFs containing Lewis basic transition metal hydroxide groups show excellent performance for trace CO2 capture via a CO2/HCO3– chemisorption mechanism and cooperative hydrogen bonding interactions reminiscent of secondary coordination sphere interactions in α-carbonic anhydrase.
Recent Publications
Catalytic Activity of a Zr MOF Containing POCOP-Pd Pincer Complexes. Kassie, A. A.; Wade, C. R. Organometallics. 2020, 39, 2214-2221. (Link)
Insights into CO2 Adsorption in M-OH Functionalized MOFs. Cai, Z.; Bien, C. E.; Liu, Q.; Wade, C. R. Chem. Mater. 2020, 32, 4257-4264. (Link)
Assessing the Role of Metal Identity on CO2 Adsorption in MOFs Containing M-OH Functional Groups. Bien, C. E.; Liu, Q.; Wade, C. R. Chem. Mater. 2019, 32, 489-497. (Link)
Synthesis and Reactivity of Zr MOFs Assembled from PNNNP-Ru Pincer Complexes. Kassie, A. A.; Duan, P.; Gray, M. B.; Schmidt-Rohr, K.; Woodward, P.; Wade, C. R. Organometallics. 2019, 38, 3419-3428. (Link)
Unveiling Reactive Metal Sites in a Pd Pincer MOF: Insights into Lewis Acid and Pore Selective Catalysis. Reiner, B. R.; Kassie, A. A.; Wade, C. R. Dalton Trans. 2019, 48, 9588-9595. (Link)
Bioinspired Metal-Organic Framework for Trace CO2 capture. Bien, C. E.; Chen, K. K., Chien, S.-C., Reiner, B. R.; Lin, L.-C.; Wade, C. R.*; Ho, W. S. W. J. Am. Chem. Soc. 2018, 140, 12662-12666. (Link)
Zirconium Metal-Organic Frameworks Assembled from Pd and Pt PNNNP Pincer Complexes: Synthesis, Postsynthetic Modification, and Lewis Acid Catalysis. Reiner, B. R.; Mucha, N. T.; Rothstein, A.; Temme, J. S.; Duan, P.; Schmidt-Rohr, K.; Foxman, B. M.; Wade, C. R.* Inorg. Chem. 2018, 57, 2663-2672. (Link)
Improved Catalytic Activity and Stability of a Palladium Pincer Complex by Incorporation into a Metal-Organic Framework. Burgess, S. A.; Kassie, A.; Baranowski, S. A.; Fritzsching, K. J.; Schmidt-Rohr, K.; Brown, C. M.; Wade, C. R. J. Am. Chem. Soc. 2016, 138, 1780-1783. (Link)
