The CBC Mass Spectrometry Facility is located in CBEC 073. We support the mass spec needs of the northern part of OSU's campus and focus on characterization services for the synthesis groups within the Department of Chemistry and Biochemistry.
Our instrumentation is utilized by researchers within the Department of Chemistry and Biochemistry, other OSU departments/colleges, other colleges/universities within Ohio, and local industry R&D.
Bruker Elute SP HPLC with Bruker Impact II QqTOF Mass Spectrometer
Analysis of small molecules, peptides, proteins

Mass Spectrometer Specs:
- Mass Range: 50 - 3000 m/z
- Mass Accuracy < 3 ppm regularly
- Electrospray Ioniziation (ESI) Source; APCI Source available with advanced notice
- Collision Cell with N2 flow for Fragmentation (MS/MS or MS2); Multiple modes available to track multiple parent/daughter ions of interest
- Set up to run Direct Injection and LC/MS modes
HPLC Specs:
- System includes autosampler, pump, column oven
- Autosampler compatible 2 mL vials, 96-well plates
- Pump Flow Rate: 0.01 – 5 mL/min, 700 bar pressure max.
- Column Oven Range: 10°C above ambient to 90°C with 1°C increments
Currently operating via submitted samples only (no walk-up access)
Bruker Microflex LRF Walk-Up Instrument
Analysis of biopolymers (peptides, proteins, DNA, RNA, oligosaccharides), large organic macromolecules, synthetic polymers,

- Time-of-Flight MS with Reflectron and Linear Modes
- 337 nm Nitrogen Laser
- Mass Range: Reflectron Mode: Up to 20,000 m/z; Linear Mode: Up to 500,000 m/z
- Resolution:
Reflectron Mode:
> 15,500 for small peptides (i.e. Somatostatin 28 at 3147.5 Da)
Linear Mode:
> 3,200 for small peptides (i.e. Bombesin at 1619.82 Da)
> 1200 for smaller proteins (i.e. Cytochrome C at 12.3 kDa)
> 495 for larger proteins (i.e. Protein A at 44.6 kDa)
Mass Accuracy:
Reflectron Mode: Internal Calibration: < 3 ppm error; External Calibration: < 25 ppm error
Linear Mode: Internal Calibration: < 45 ppm error, External Calibration: < 50 ppm error
Limits of Detection:
Reflectron Mode: S/N ~ 25 for 1 fmol Glu-Fib (1570.7 Da)
Linear Mode: S/N > 130 for 500 fmol BSA (66 kDa)
Thermo Orbitrap Exploris MX LC-MS Walk-Up Instrument
Analysis of small molecules, peptides, proteins, metalloproteins

Mass Spectrometer Specs:
- Mass Range: 40 - 3000 m/z
- Mass Accuracy < 1 ppm regularly with Easy-IC Calibrant
- Electrospray Ioniziation (ESI) Source; APCI Source available with advanced notice
- Resolving power up to 180,000 for high resolution analyses
HPLC Specs:
- Vanquish UHPLC System includes autosampler, binary pump , column oven
- Six solvent channels to select from
- Autosampler compatible with 2 mL vials, 96-well plates; temperature control available
- Pump Flow Rate: 0.001 – 8 mL/min, 1000 bar pressure max.
- Column Oven Range: 10°C to 120°C with 0.01°C increments; solvent pre-heater with adjustable temps to match column oven temp
2023
Orbitrap Exploris MX instrument installed Fall 2023!
Now available for use as a walk-up instrument!
2020
Bruker Elute SP HPLC with Bruker Impact II QqTOF Mass Spectrometer Installed
Direct Injection and LC/MS modes - for MS, MS/MS, MS2
Bruker Microflex LRF Installed
Improved Mass range and resolution!
Interested in Utilizing Instrumentation in the MSF?
Bruker Impact: Submitted samples only. Users will undergo a short training on sample prep/retrieving data.
Bruker MALDI: Users will be trained on instrument operation and given 24/7 walk-up access.
Orbitrap: Users will be trained on instrument operation and given 24/7 walk-up access.
OSU Students/Staff:
- Make sure you have an active FOM account (fom.osu.edu). This is the site used for instrument reservations and billing.
- Create a requisition through Workday following the instructions on this website. Please select "CBC Mass Spectroscopy Facility" as the supplier. Check the rates tab above to help determine the total $ amount you will want to submit to cover your expected experiments.
- Once the requisition has been fully approved through the Workday and you receive an email notification of approval, manually add the requisition to your FOM account:
- Choose "My Accounts" link on the left-hand side towards the bottom of the screen.
- Go to the "Add a New Account" section partway down the page. Fill in the boxes for Research Description Name and Workday Requisition Number, click "Add this Account".
- You should receive a pop-up confirmation that the RQ was added successfully. If not, double check by signing out, waiting a few minutes, signing back in, and checking "My Accounts" to see if it is now listed. If not, please attempt to add it again. Note: requisitions cannot be added until they have been fully approved in Workday (workflow completed).
- Request access/training to the instrumentation on FOM. Please list types of samples/experiments you would like to analyze and best days/times for scheduling training sessions.
External Researchers:
Please email Dr. Alicia Friedman at friedman.270@osu.edu.
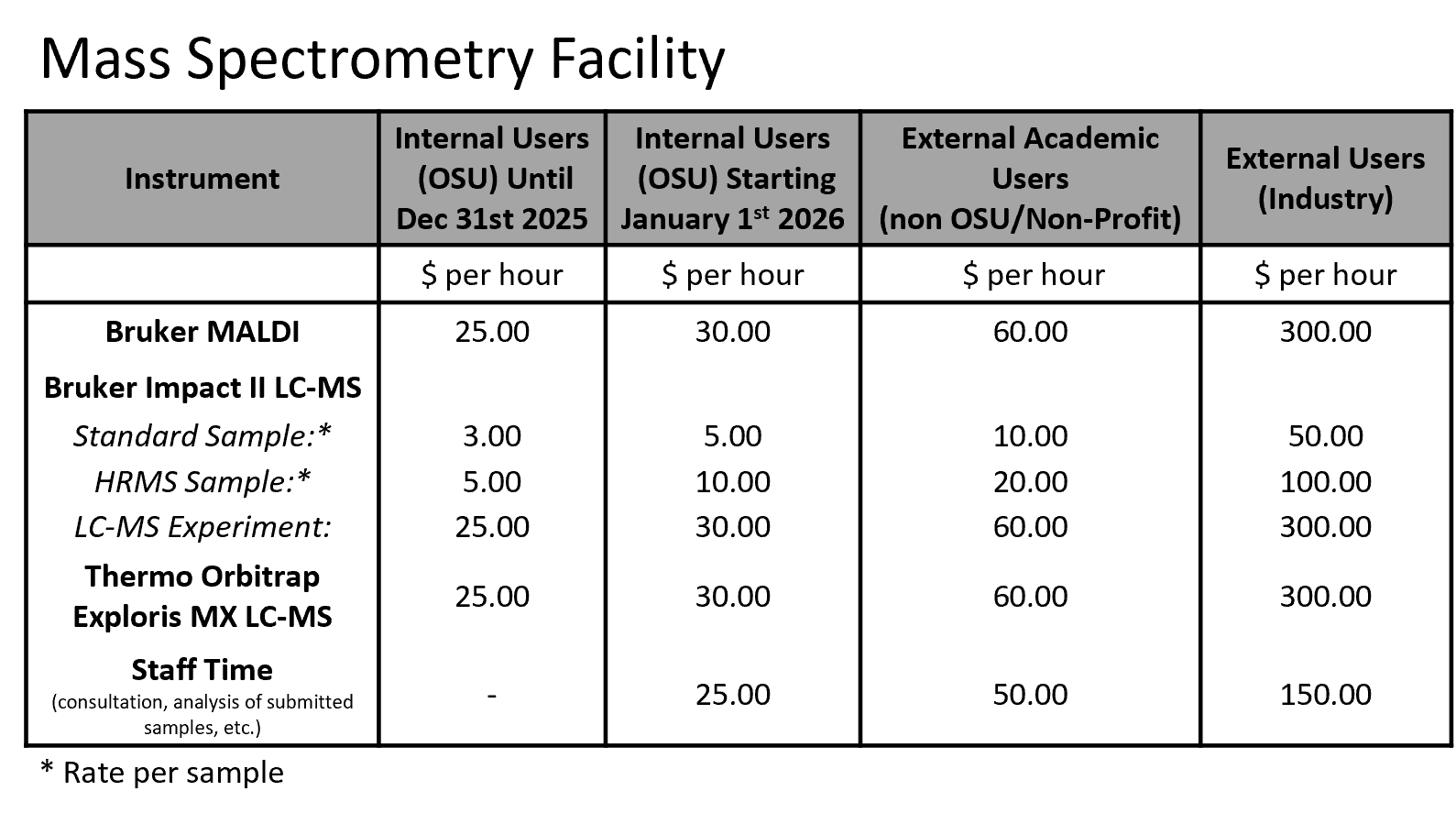
Rates are current as of 7/1/2024
Mass Spec Facility Policies
1. Users are expected to follow all RSS, Department of Chemistry & Biochemistry, and university policies. The policies below are specific to the MSF.
2. All users are expected to follow good laboratory practices in the handling of chemicals, waste, and instrumentation. Users must follow all policies and directions of MSF staff such as posted notices, emails, verbal instructions, etc.
3. All injuries, accidents, near-misses, fires, safety issues, etc. are to be reported immediately to the facility manager (Dr. Alicia Friedman, friedman.270@osu.edu, 614-247-7814, CBEC 085) and the CBC Chemical Safety Coordinator (Dr. Amy Moore, moore.4061@osu.edu, 614-292-3897, CBEC 163)
4. All new users must complete the facility "New User Safety Checklist" during training. This is kept in the safety binder in the lab for EHS to access quickly/easily.
5. Be aware of the nearest fire extinguisher/alarm, safety eyewash station, sink, and evacuation routes to exit the building during an emergency.
6. The Hazardous Communication Plan is always available in a binder on the front desk near the door.
7. No food/drinks are to be brought into the facility at any time.
8. PPE Requirements:
- Safety eyewear (i.e. safety glasses) must be worn at all times in the facility
- Dress appropriately – no sandals, no shorts allowed
- Gloves are provided and to be used at your own discretion – do not use gloves when using the mouse/keyboard of a computer!
- Lab coats are only required when working with hazardous materials
9. Waste must be disposed of properly and in accordance with university policy:
- Glass waste bins are clearly labeled – do not include anything not glass (i.e. gloves, Kimwipes, etc.)
- Liquid waste containers are clearly labeled – do not pour incompatible solvents down the drain.
- Sharps bins are provided and clearly labeled – do not recap sharps as to avoid accidental sticks!
10. Sample Limitations:
- No radioactive materials are permitted in the facility
- No biohazards that require a lab safety of BSL are permitted in the facility – these include infectious viruses, toxins, human tissues, viable plasmids, etc.
11. Users should not remove or tamper with any part of an instrument unless instructed to do so by a staff member. If an instrument is not working, please report it to Dr. Friedman immediately!
12. Instruments are to be used only by trained/approved individuals.
- Untrained individuals are welcome to accompany a trained user to observe the experiment but are not to operate any of the software/hardware of the instrument
- Training must take place with a MSF staff member prior to being granted access. The overall number and length of training sessions depends on the individual instrument, planned experiment, and trainee’s previous experience.
13. All instrument time must be reserved and logged using the FOM system (fom.osu.edu). This includes any time using the computer for data retrieval and/or data workup.
- RSS policy states that users who do not accurately self-report instrument usage will be charged from the time the log-on occurred until the next legitimate user log-on – this can get quite pricey if an instrument is not regularly used or occurs over a weekend!
- Repeat infractions will result in loss of access to the facility and instruments
14. Facility computers and data drives are not regularly backed up; data may be removed (with prior notice) if space is needed. Users are expected to transfer all experiments to personal/lab data storage that is secure and regularly archived.
15. These policies are subject to change with notice at any time.
Staff

MSF Lab: CBEC 073
Office: CBEC 085
614-247-7814
friedman.270@osu.edu

Carson Ward (Graduate TA)
ward.1648@osu.edu
Graduate Student
B.S. Chemistry, Ohio University (2021)