T. V. (Babu) RajanBabu
Contact Information
Arts and Sciences Distinguished Professor Emeritus
Areas of Expertise
- Organic
Bio
RajanBabu received his B. Sc (Special) and M. Sc. degrees from Kerala University and The Indian Institute of Technology (IIT, Madras) respectively. He obtained a Ph. D. degree from The Ohio State University working with Professor Harold Shechter, and was a postdoctoral fellow at Harvard University with the late Professor R. B. Woodward. He then joined the Research Staff of Dupont Central Research and Development becoming a Research Fellow in 1993. He returned to Ohio State as a Professor of Chemistry in 1995 and is currently a Distinguished Professor in the College of Arts and Sciences.
Research Overview
New Methods for Stereoselective Synthesis, Enantioselective Catalysis, Multi-component Cyclization Methods, Free Radical Chemistry, Natural Product Synthesis
We are engaged in two major areas of research that deals with new methodology for stereoselective synthesis: (a) enantioselective catalysis of C-C, C-H and C-N bond formations; (b) novel multi-component cyclization methods that lead to uncommon, atropisomeric 1,3-diene derivatives. These intermediates are formed with vinyl Si, Sn or B moieties, which make them useful for further stereoselective transformations. We are also pursuing applications of the newly developed methods for the synthesis of biologically relevant molecules.
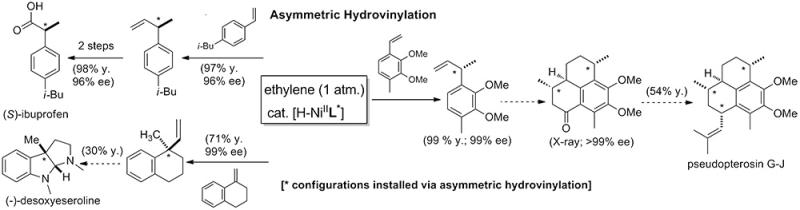
In the asymmetric catalysis area, we recently developed practical methods for C-C bond-formations based on hydrovinylation of vinylarenes, 1,3-dienes and strained olefins. Applications of this chemistry include a new highly enantioselective synthesis of popular 2-arylpropionic acids such as (S)-ibuprofen, and a new approach to controlling the exocyclic side-chain stereochemistry in molecules such as pseudopterosins, deoxyeseroline (a molecule containing an all-carbon quaternary asymmetric center) and side-chains of steroid D-ring substituents.
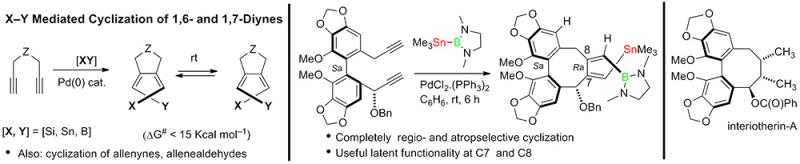
Examples of multi-component addition/cyclization of diynes (giving novel axially chiral 1,3-dienes), and a prototypical highly functionalized dibenzocyclooctadiene that has been synthesized by this chemistry are shown below. In other published work we have shown that related cyclization of allene-aldehydes allows expeditious entry into highly alkylated indolizidine alkaloids such as IND-223A.
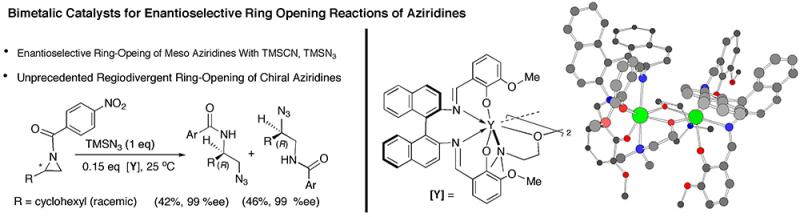
Other areas of our research interests are, coordination catalysis (asymmetric ring opening reactions of epoxides and aziridines), asymmetric hydrocyanation, new free radical cyclization methods, development of organometallic reagents for aqueous organic chemistry, and synthesis of feedstock chemicals from renewable sources.
External support for our research comes primarily from by the National Science Foundation and the National Institutes of Health.
Former members of our group hold faculty positions at Nagoya University (Japan), University of North Carolina (Chapel Hill), Hanyang University (Korea), Indian Institute of Technology (Rajasthan); National Changhua University of Education (Taiwan); research positions at National Institute of Health, Federal Drug Administration as well as industrial positions at Abbott Pharmaceuticals, Albany Molecular, Boehringer-Ingelheim, Firestone, Manville, Novartis, Pharmacore and Sanofi Aventis.
I recruit 1-3 graduate students each academic year and welcome inquiries regarding these openings.
Recent Publications
For a complete list of publications, use this link.
“Activator-free single-component Co(I)-catalysts for regio- and enantioselective heterodimerization and hydroacylation reactions of 1,3-dienes. New reduction procedures for synthesis of LCo(I)-complexes and comparison to in situ generated catalysts.” Parsutkar, M. M.; Moore, C. E.; RajanBabu, T. V Dalton Trans. 2022, 51, 10148-10159. DOI: 10.1039/d2dt01484j
“gamma C-H Functionalization of Amines via Triple H-Atom Transfer of a Vinyl Sulfonyl Radical Chaperone’, Herbort, J. H.; Bednar, T. N.; Chen, A. D.; RajanBabu, T. V.; Nagib, D. A. J. Am. Chem. Soc. 2022, 144, 13366-13373. DOI: 10.1021/jacs.2c05266
"Reactions of Epoxides Mediated by Low-Valent Titanium Reagents", Nugent W. A; Halder, S.; RajanBabu, T. V. . Organic Reactions 2022, 111, xxxx-xxxx (in press).
"Catalytic Enantioselective Hydrovinylation of Trialkylsilyloxy and Acetoxy-1,3-Dienes: Cationic Co(I) Complexes for the Synthesis of Chiral Enolate Surrogates and Their Applications for Synthesis of Ketones and Cross-Coupling Reagents in High Enantiomeric Purity," Biswas, S.; Dewese, K. R.; Raya, B.; RajanBabu, T. V. ACS Catal. 2022, 5094-5111. DOI: 10.1021/acscatal.2c00546.
“A New Paradigm in Enantioselective Cobalt Catalysis: Cationic Cobalt(I) Catalysts for Heterodimerization, Cycloaddition, and Hydrofunctionalization Reactions of Olefins”, Biswas, S.; Parsutkar, M. M.; Jing, S. M.; Pagar, V. V.; Herbort, J. H.; RajanBabu, T. V Acc. Chem. Res. 2021, 54, 4545-4564. DOI: 10.1021/acs.accounts.1c00573.
"α- and β-Functionalized Ketones from 1,3-Dienes and Aldehydes: Control of Regio- and Enantioselectivity in Hydroacylation of 1,3-Dienes." Parsutkar, M. M.; RajanBabu, T. V., J. Am. Chem. Soc. 2021, 143, 12825-12835. DOI: 10.1021/jacs.1c06245.
"Cationic Co(I) Catalysts for Regiodivergent Hydroalkenylation of 1,6-Enynes: An Uncommon cis-β-C–H Activation Leads to Z-Selective Coupling of Acrylates," Herbort, J. H.; Lalisse, R. F.; Hadad, C. M.; RajanBabu, T. V., ACS Catal. 2021, 11, 9605-9617. DOI: 10.1021/acscatal.1c02530.
“Four Mechanistic Mysteries: The Benefits of Writing a Critical Review”, Nugent, W. A.; RajanBabu, T. V. Angew. Chem. Int. Ed. 2021, 60, 2194-2201. DOI: 10.1002/anie.202011838.
“Mechanism of Cobalt-Catalyzed Heterodimerization of Acrylates and 1,3-Dienes. A Potential Role of Cationic Cobalt(I) Intermediates”, Gray, M.; Hines, M. T.; Parsutkar, M. M.; Wahlstrom, A. J.; Brunelli, N. A.; RajanBabu, T. V. ACS Catal 2020, 10, 4337-4348. doi: 10.1021/acscatal.9b05455.
“Catalytic Enantioselective Synthesis of Cyclobutenes from Alkynes and Alkenyl Derivatives”, Parsutkar, M. M.; Pagar, V. V.; RajanBabu, T. V. J. Am. Chem. Soc. 2019, 141, 15367-15377. doi: 10.1021/jacs.9b07885.
“Cationic Co(I)-Intermediates for Hydrofunctionalization Reactions: Regio- and Enantioselective Cobalt-Catalyzed 1,2-Hydroboration of 1,3-Diene”, Duvvuri, K.;. Dewese, K. R.; Parsutkar, M. M.; Jing, S. M.; Mehta, M. M.; Gallucci, J. C.; RajanBabu, T. V. J. Am. Chem. Soc. 2019, 141, 7365-7375. doi: 10.1021/jacs.8b13812
"Broadly Applicable Stereoselective Syntheses of Serrulatane, Amphilectane Diterpenes, and Their Diastereoisomeric Congeners Using Asymmetric Hydrovinylation for Absolute Stereochemical Control", Tenneti, S.; Biswas, S.; Cox, G. A.; Mans, D. J.; Lim, H. J.; RajanBabu, T. V. J. Am. Chem. Soc. 2018, 140, 9868-9881.
"Tandem catalysis for asymmetric coupling of ethylene and enynes to functionalized cyclobutanes", Pagar, V. V.; RajanBabu, T. V. Science 2018, 361, 68-72.
"Catalytic Enantioselective Hetero-dimerization of Acrylates and 1,3-Dienes", Jing, S. M.; Balasanthiran, V.; Pagar, V.; Gallucci, J. C.; RajanBabu, T. V. J. Am. Chem. Soc. 2017, 139, 18034-18043.
"Conformation and reactivity in dibenzocyclooctadienes (DBCOD). A general approach to the total synthesis of fully substituted DBCOD lignans via borostannylative cyclization of alpha,omega-diynes", Gong, W.; RajanBabu, T. V. Chem. Sci. 2013, 4, 3979-3985.
“Asymmetric Hydrovinylation of 1-Vinylcycloalkenes. Reagent Control of Regio- and Stereoselectivity”, Page, J. P.; RajanBabu, T. V. .J. Am. Chem. Soc. 2012, 134, 6556-6559.
“Asymmetric Hydrovinylation of Vinylindoles. A Facile Route to Cyclopenta[g]indole Natural Products (+)-cis-Trikentrin A and (+)-cis-Trikentrin B” Liu, W.; Lim, H. J.; RajanBabu, T. V. J. Am. Chem. Soc. 2012, 134, 5496-5499.
“On the Stereochemistry of Acetylide Additions to Highly Functionalized Biphenylcarbaldehydes and Multi-component Cyclization of 1,n-Diynes. Syntheses of Dibenzocyclooctadiene Lignans”, Gong, W.; Singidi, R. R.; Gallucci, J. C.; RajanBabu, T. V. Chem. Sci. 2012, 3, 1221–1230.
“Phosphinite and Phosphonite Ligands”, in Phosphorus (III) Ligands in Homogeneous Catalysis- Design and Synthesis, Paul C. J. Kamer, Piet W. N. M. van Leeuwen, Eds. Wiley: London, 2012, pp. 159–232.
“Enantioselective Hydrovinylation of Alkenes”, RajanBabu, T. V. in Comprehensive Chirality, Yamamoto, H.; Carreira E., Eds. Elsevier: London, 2011.
"Ethylene in Organic Synthesis. Repetitive Hydrovinylation of Alkenes for Highly Enantioselective Syntheses of Pseudopterosins", Mans, D. J.; Cox, G. A.; RajanBabu, T. V. J. Am. Chem. Soc. 2011, 133, 5776–5779.
“Stereoselective Cyclization of Functionalized 1,n-Diynes Mediated by [X-Y]- Reagents [X-Y = R₃Si-SnR’₃ or (R₂N)₂B-SnR’₃]. Synthesis and Properties of Atropisomeric 1,3-Dienes”, Singidi, R. R.; Kutney, A. M.; RajanBabu, T. V. J. Am. Chem. Soc.2010, 132, 13078-13087.
"Asymmetric Hydrovinylation of Unactivated Linear 1,3-Dienes", Sharma, R. K;. RajanBabu, T. V. J. Am. Chem. Soc. 2010, 132, 3295-3297.
"Regiodivergent Ring Opening of Chiral Aziridines", Wu, B.; Parquette, J. R.; RajanBabu, T. V. Science 2009, 326, 1662.
"Enantioselective Desymmetrization of meso-Aziridines by TMSN₃ and TMSCN Catalyzed by Discrete Yttrium Complexes"", Wu, B.; Parquette, J. R.; RajanBabu, T. V. Angew. Chem. Int. Ed. Engl. 2009, 48, 1126-1129.
"Conformationally Driven Asymmetric Induction in a Catalytic Dendrimer", Yu, J.; RajanBabu, T. V.; Parquette, J. R. J. Am. Chem. Soc. 2008, 130, 7845-7847.
"Ligand Tuning in Asymmetric Hydrovinylation of 1,3-Dienes. A Stereoselective Route to either Steroid-C20(S) or -C20(R) Derivatives", Saha, B.; Smith, C. R.; RajanBabu, T. V. J. Am. Chem. Soc. 2008, 130, 9000-9005.
"All Carbon Quaternary Centers via Asymmetric Hydrovinylation. New Approaches to the Exocyclic Side Chain Stereochemistry Problem", Zhang, A.; RajanBabu, T. V. J. Am. Chem. Soc. 2006, 128, 5620-5621.
"Hydrovinylation of 1,3-Dienes. A New Protocol, an Asymmetric Variation, and a Potential Solution to the Exocyclic Side Chain Stereochemistry Problem", Zhang, A.; RajanBabu, T. V. J. Am. Chem. Soc. 2006, 128, 54-55.
A Review of recent research:“Hydrovinylation Reactions in Organic Synthesis”, Comprehensive Organic Synthesis, 2nd edition, Vol. 5, Oxford: Elsevier; 2014. pp. 1582-1620. DOI: 10.1016/B978-0-08-097742-3.00533-4.
