
Vicki Wysocki
Ohio Eminent Scholar
279 Biomedical Research Tower
460 W 12th Ave
Columbus, OH 43210
Areas of Expertise
- Analytical
- Biochemistry
Bio
Vicki Wysocki received her BS in Chemistry from Western Kentucky University in 1982 and her PhD in Chemistry from Purdue University in 1987. After pursuing postdoctoral studies at Purdue and at the Naval Research Laboratory as a National Research Council Fellow, she joined Virginia Commonwealth University as an Assistant Professor in 1990. She was promoted to Associate Professor in 1994. Vicki joined the University of Arizona in 1996, was promoted to Professor in 2000, and served as co-chair and chair of the Department of Chemistry and Biochemistry 2008-2012. Vicki joined OSU in August 2012 as an Ohio Eminent Scholar and Director of the OSU Campus Chemical Instrument Center. Awards include the 2009 Distinguished Contribution to Mass Spectrometry Award from the American Society for Mass Spectrometry, jointly with Professor Simon Gaskell, the 2017 American Chemical Society Field and Franklin Award for Outstanding Contributions to Mass Spectrometry, the 2022 ACS Division of Analytical Chemistry Award in Chemical Instrumentation, and the 2022 Thomson Medal from the International Mass Spectrometry Foundation. Vicki served as VP Programs, President, and Past President of the American Society for Mass Spectrometry from 2014-2020. She was an associate editor for the ACS journal Analytical Chemistry from 2015-2022 and is currently Editor-in-Chief of the Journal of the American Society for Mass Spectrometry.
Research Overview
Native Mass Spectrometry/ Ion-Surface Collisions /Large Protein-Protein Complexes/ Peptide and Protein Sequencing /Proteomics/Metabolomics
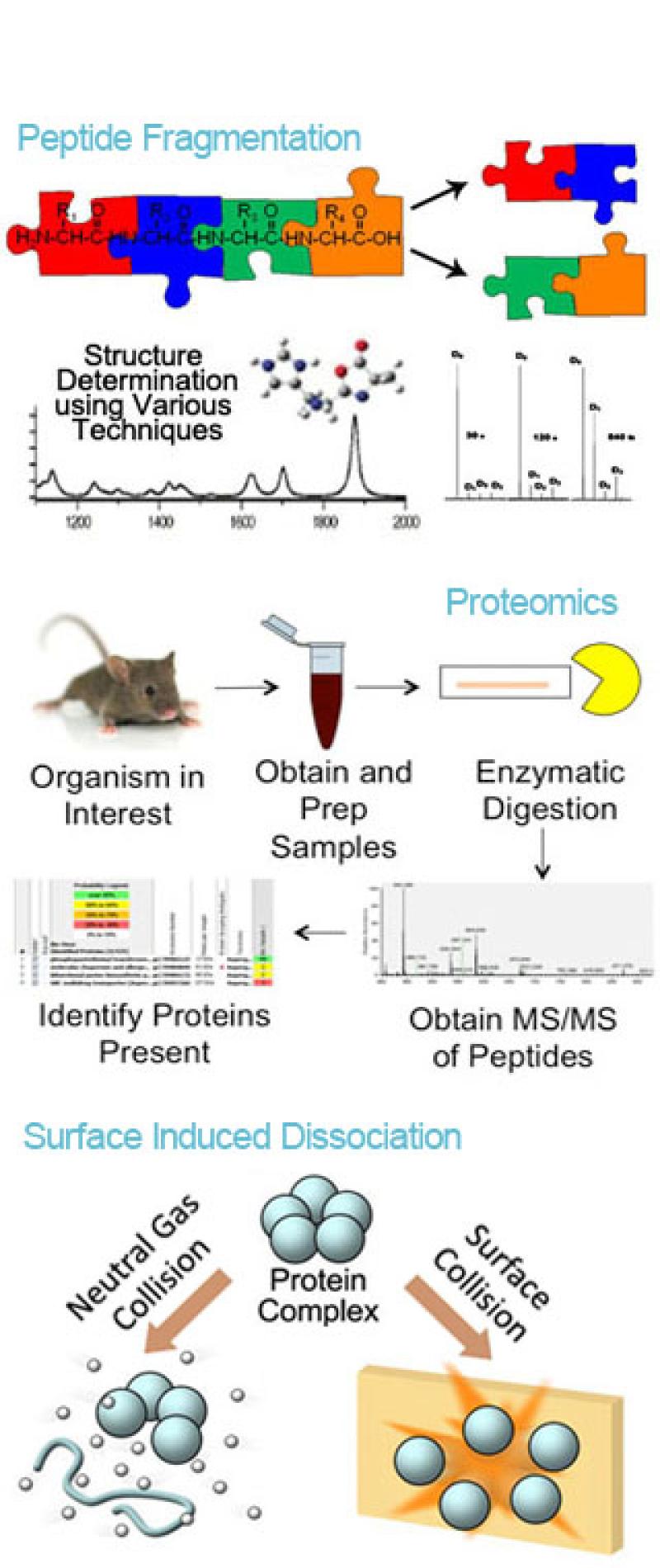
Research in the Wysocki group is categorized into four broad areas:(1) development and implementation of surface-induced dissociation onto commercial time-of-flight, Orbitrap and FT-ICR instruments, (2) development and application of native mass spectrometry-guided structural biology approaches, (3) multi-omics approaches to biomarker discovery, disease diagnosis and prognosis using proteomics and metabolomics methods coupled with genomics and transcriptomics, and (4) determination of peptide and other fragment ion structures by IR action spectroscopy.
(1) Surface-Induced Dissociation Development and Implementation
Subunit organization of protein complexes can be studied with tandem mass spectrometry by disruption of the quaternary structure in a controlled manner. The major challenge of using this method for structural analysis of non-covalent protein complexes is to overcome the undesired unfolding of subunits that occurs in the commonly used collision activation with gaseous targets, resulting in loss of original structural information. This area of research involves the development of surface-induced dissociation (SID) devices for improved structural characterization of large protein complexes. We aim to create devices that are “vendor-neutral” and allow for straightforward operation by the end-user. Our custom SID devices reveal protein complex architecture, including subunit stoichiometry and connectivity, and minimal or reduced unfolding compared with CID.
(2) Native MS of Protein and Nucleoprotein Complexes
Our NIH-funded Resource for Native MS-Guided Structural Biology develops MS and related technology (e.g., online separations and ion mobility) to build an integrated structural biology approach. Collaborations with scientists from around the world provide biological problems that drive the technology development while providing group members with collaborative science training and expertise in handling and characterization of protein, nucleoprotein, and membrane protein complexes.
(3) Proteomics and Metabolomics
A third area of research in the Wysocki lab is combined proteomics and metabolomics analysis to study different disease systems. This area of research ranges from understanding microbial ecology during Salmonella infection to decoding deregulated pathways in lung cancer. Expansion of the Salmonella project includes characterizing metabolic exchanges between Salmonella and its competitors in the gut as well as protein profiling to identify nutrient acquisition and utilization pathways that could correspond to consumption of metabolites by the microbiota. This research will facilitate improved prophylactic and therapeutic options in the future.
(4) Fragment Ion Structures by IRMPD
These projects are designed to increase the current understanding of the fragmentation patterns of activated protonated peptides and other ions (glycans, RNA/DNA). The long-range goals of this work are to provide additional "rules" that will, ultimately, relate information on gas-phase fragmentation patterns and energetics of dissociation to the gas-phase conformations of intact and fragmented molecules. The investigation of fragmentation mechanisms/product ion structure is primarily performed by combining experimental data with computationally derived structures and their theoretical infrared spectra. Experimental data are collected using free electron laser (FEL) Infrared Multiphoton Dissociation (IRMPD) spectroscopy in which a mass spectrometer is used to detect fragments as a function of infrared wavelength, generating an action IR spectrum.
Please refer to the Wysocki Group Website for more detailed descriptions about the ongoing research projects.
Recent Publications
Click here for the current publications list.
